-
PDF
- Split View
-
Views
-
Cite
Cite
Yiping Qi, Xiaohong Li, Yong Zhang, Colby G Starker, Nicholas J Baltes, Feng Zhang, Jeffry D Sander, Deepak Reyon, J Keith Joung, Daniel F Voytas, Targeted Deletion and Inversion of Tandemly Arrayed Genes in Arabidopsis thaliana Using Zinc Finger Nucleases, G3 Genes|Genomes|Genetics, Volume 3, Issue 10, 1 October 2013, Pages 1707–1715, https://doi.org/10.1534/g3.113.006270
Close - Share Icon Share
Abstract
Tandemly arrayed genes (TAGs) or gene clusters are prevalent in higher eukaryotic genomes. For example, approximately 17% of genes are organized in tandem in the model plant Arabidopsis thaliana. The genetic redundancy created by TAGs presents a challenge for reverse genetics. As molecular scissors, engineered zinc finger nucleases (ZFNs) make DNA double-strand breaks in a sequence-specific manner. ZFNs thus provide a means to delete TAGs by creating two double-strand breaks in the gene cluster. Using engineered ZFNs, we successfully targeted seven genes from three TAGs on two Arabidopsis chromosomes, including the well-known RPP4 gene cluster, which contains eight resistance (R) genes. The resulting gene cluster deletions ranged from a few kb to 55 kb with frequencies approximating 1% in somatic cells. We also obtained large chromosomal deletions of ~9 Mb at approximately one tenth the frequency, and gene cluster inversions and duplications also were achieved. This study demonstrates the ability to use sequence-specific nucleases in plants to make targeted chromosome rearrangements and create novel chimeric genes for reverse genetics and biotechnology.
Genome sequences of many plant species have been completed, and all contain a significant number of tandemly arrayed genes (TAGs). For example, approximately 14% of rice genes (International Rice Genome Sequencing Project 2005), 17% of Arabidopsis genes (Arabidopsis Genome Initiative 2000), and 35% of maize genes (Messing et al. 2004) are organized in tandem. The genetic redundancy resulting from TAGs presents a challenge for reverse genetics (Jander and Barth 2007). It is difficult, if not impossible, to eliminate TAG expression with the use of methods such as ethyl methanesulfonate mutagenesis and targeting-induced local lesions in genomes, i.e., TILLING (Koornneef et al. 1982; McCallum et al. 2000), T-DNA and transposon insertional mutagenesis (Alonso et al. 2003; Raina et al. 2002; Rosso et al. 2003; Sessions et al. 2002; Woody et al. 2007), or RNA interference and miRNA-based gene silencing (Abbott et al. 2002; Alvarez et al. 2006; Hamilton and Baulcombe 1999; Schwab et al. 2006). One promising approach for studying TAGs is to make chromosomal deletions. Ionizing radiation, however, acts randomly (Li et al. 2001), making it difficult to recover the desired deletion. Although the Cre-Lox system has proven effective for making deletions, it relies on large LoxP T-DNA insertion populations (Zhang et al. 2003), which currently are unavailable for most plant species.
An alternative approach to make targeted genome deletions is to use sequence-specific nucleases. These proteins, which include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and meganucleases, make site-specific DNA double-strand breaks (DSBs) at a locus of interest (Christian et al. 2010; Kim et al. 1996; Smith et al. 2006). Repair of DSBs occurs by two pathways, namely nonhomologous end-joining (NHEJ) and homologous recombination (HR) (Puchta 2005; Puchta et al. 1996). NHEJ is error-prone and typically leads to insertions, deletions (indels), and substitutions at the cleavage site. In contrast, repair by HR is typically error-free because it uses a DNA template to correct the break. Of the three nuclease platforms, ZFNs have been most widely used in plants. ZFNs have been successfully used for targeted mutagenesis by NHEJ in Arabidopsis (Osakabe et al. 2010; Zhang et al. 2010) and soybean (Curtin et al. 2011), as well as for gene targeting by HR in tobacco (Townsend et al. 2009) and maize (Shukla et al. 2009). In addition, Petolino et al. (2010) reported that when a 4.3-kb beta-glucuronidase transgene was flanked by two ZFN sites, it could be efficiently deleted from the tobacco genome, thus demonstrating that ZFNs can induce chromosomal deletions of transgenes in plants. Although ZFN-mediated deletion, inversion, and duplication of endogenous chromosomal DNAs has been achieved in human cells (Lee et al. 2010, 2011), none of these chromosome rearrangements have yet to be demonstrated in plant cells by the use of sequence-specific nucleases.
In Arabidopsis, the receptor-like kinase (RLK) and the nucleotide-binding and leucine-rich repeat resistance (R) gene families are large and have ~600 and ~150 gene members, respectively (Meyers et al. 2003; Shiu et al. 2004). Both gene families play important roles in plant development and immunity. For example, many plant hormone receptors and almost all plant immune receptors are members of these two families. Genes in both families are organized in tandem throughout the genome. In this study, we sought to delete endogenous TAGs by using ZFNs that target three RLK gene clusters and one large R gene cluster. We successfully demonstrated targeted deletions, inversions, and duplications of multiple gene clusters as well as large chromosomal deletions exceeding 9 Mb.
Materials and Methods
ZFN assembly
Genomic DNA sequences of target genes were analyzed with the software ZiFiT Targeter (version 3.3) to identify ZFN sites for which ZFNs could be engineered using the Context-Dependent Assembly (CoDA) method (Curtin et al. 2011; Sander et al. 2011). DNA sequences encoding ZFNs of choice (Supporting Information, Figure S1 and Table S1) were assembled by mutagenesis and overlapping polymerase chain reaction (PCR) using standard molecular cloning procedures. For each ZFN, ZF arrays were first cloned into the yeast expression vectors pCP3 and pCP4 using available XbaI and BamHI sites (Zhang et al. 2010). Then, DNA sequences for the left and right ZF arrays were excised from the yeast expression vectors with XbaI and BamHI and moved into the pZHY013 entry clone using the XbaI-BamHI and NheI-BglII sites, respectively (Figure S2). pZHY013 contains an obligate FokI heterodimer architecture (Miller et al. 2007), and the ZFNs are linked by a T2A translational skipping sequence. The plant ZFN expression vectors were made using a Gateway LR reaction between the aforementioned entry clones and the pFZ19 destination vector (Zhang et al. 2010).
Transgenic plants and expression of ZFNs
Agrobacterium tumefaciens GV3101/pMP90 was transformed with pFZ19 plasmids containing the ZFNs. The transformed A. tumefaciens strain was then used to transform Arabidopsis Col-0 (wild-type) plants using the floral dip method (Clough and Bent 1998). T1 transgenic plants were selected by growing the sterilized seeds on 0.5× MS solid medium (0.8% agar) that contained 100 μg/mL timentin (PlantMedia) and 20 μg/mL hygromycin B (Roche). For inducing ZFN expression, 20 µM β-estradiol (Sigma-Aldrich) was included in the medium.
ZFN activity measurement
One-week-old seedlings grown on MS medium with estradiol were harvested for DNA extraction with the CTAB DNA isolation method (Stewart and Via 1993). Eight T2 transgenic plants from the same T1 parent were bulked to represent each sample, whereas eight wild-type plants were bulked as the negative control.
To detect ZFN activity, an enrichment PCR procedure was used. To summarize, ~500 ng of genomic DNA from each sample was digested overnight (16 hr) with 1 µL of DdeI (for At1g53-ZFN), AflII (for At1g70-ZFN), BfaI (for At4g16-ZFN), BmgBI (for At3g21-ZFN), or EarI (for At5g01-ZFN) in a 20-µL reaction volume. Four microliters of digested genomic DNA was used for PCR amplification of the corresponding ZFN target sites in a 25-µL reaction volume. Ten microliters of unpurified PCR product was then digested with 1 µL of the same restriction enzyme in a 40-µL reaction volume for 12−16 hr. Digested products were resolved by electrophoresis in 1.5% agarose gels; mutations created by ZFNs were evidenced as undigested PCR products.
An alternative method to detect and measure ZFN activity by restriction digestion is similar to enrichment PCR except that the genomic DNA digestion step is omitted. Four microliters of 50 ng/µL genomic DNA was directly used for PCR and subsequent digestion by the corresponding restriction enzyme. The frequency of ZFN-mediated mutagenesis was measured by quantifying the percentage of undigested PCR product that was resolved by electrophoresis on a 1.5% agarose gel.
ZFN activity also was measured by the Surveyor assay in which T7 endonuclease was substituted for Cel-I (Guschin et al. 2010). In summary, PCR products amplified from genomic DNA templates were purified with a QIAquick PCR purification kit. For each sample, ~500 ng of purified PCR product was mixed with NEB buffer 2 in a 30-µL volume. To promote heteroduplex formation, PCR amplicons were denatured and reannealed using the following regime: 95° for 5 min, 95° to 85° at −1.5°/sec, and 85° to 25° at −0.1°/sec. One microliter of T7 endonuclease (NEB) was added to each sample for digestion at 37° for 1 hr. The digested products were resolved by electrophoresis in 1.5% agarose gels, and the frequency of ZFN-mediated mutagenesis was quantified as described previously (Guschin et al. 2010).
Sequence confirmation of mutagenesis and deletions
Both undigested PCR products (for assessing mutagenesis) and PCR products (for detecting deletions, inversions, and duplications) were purified with the QIAquick Gel Extraction Kit. The purified DNA products were then cloned using either the pCR8/GW/TOPO TA Cloning Kit or the pCR2.1 Original TA Cloning Kit (Invitrogen). Multiple clones for each experiment were randomly picked and subjected to DNA sequencing.
Deletion frequency measurements
A method similar to digital PCR analysis (Lee et al. 2010) was used to estimate deletion frequencies. Genomic DNA samples were serially diluted (in a 3× gradient) in distilled water, and they were used for PCR via the use of deletion-specific primer pairs (Table S2). The same DNA samples were used to amplify a fragment of the ADH1 gene as a genomic DNA copy number control. In each case, the difference of dilution factors for both deletion PCR and control PCR was used to calculate deletion frequency.
Results
Strategy for targeted deletion of TAGs
Chromosomal deletions can be stimulated by two coordinated DSBs (Petolino et al. 2010). In our strategy, we used a single pair of ZFNs, which because of the high sequence similarity among TAGs, created two or more DSBs. Five ZFNs were engineered using the CoDA method to target three RLK gene clusters, the RPP4 R gene cluster, and the ASK8 gene cluster (Figure 1 and Figure S3). These five ZFNs were expected to induce a total of 13 DNA DSBs in targeted exons of the Arabidopsis genome. The resulting size of predicted deletions ranged from a few to more than 50 kb. In addition to inducing deletions, ligation of broken chromosomes can create novel gene fusions. However, because NHEJ is error-prone, we anticipated that indels would be introduced at the cleavage site, thus rendering some of the deleted TAGs nonfunctional.
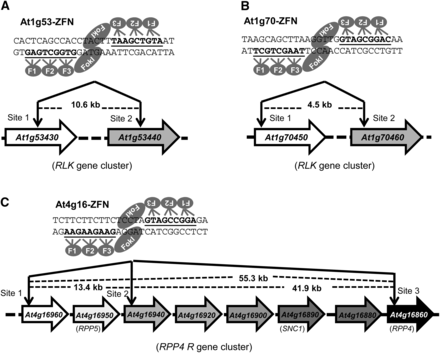
Schematic of target genes and ZFN sites. (A) The At1g53-ZFN targets both At1g53430 and At1g53440 in the 14th exon of each gene. (B) The At1g70-ZFN targets At1g70450 in the 1st exon and At70460 in the 2nd exon. (C) The At4g16-ZFN targets three sites in the RPP4 R gene cluster: the 1st exon of At4g16960, the 5′ UTR of At4g16940, and the 1st exon of At4g16860. Illustration of ZFN pairs depicts the DNA recognition triplets for each zinc finger. The zinc finger binding sequences are underlined, and the distance between cleavage sites is shown.
Detection of ZFN-induced mutagenesis
For all ZFNs used in this study, both the left and right ZFNs were expressed from an estradiol-inducible promoter and separated by a “self-cleaving” T2A peptide, which promotes production of two proteins from one mRNA by a translational skipping mechanism (Szymczak et al. 2004). T1 transgenic plants were screened on MS medium containing both hygromycin (to identify transgenic plants) and β-estradiol, which allowed for induction of ZFN transgenes. Detection of ZFN-induced mutations was performed by the use of enrichment PCR (Qi et al. 2013). Indels introduced by ZFNs frequently occur in the “spacer” region, where the FokI nuclease domains dimerize and cleave the DNA. Thus, if there is a unique restriction enzyme site in the spacer, this restriction enzyme site will likely be destroyed through ZFN-mediated mutagenesis and thus render the DNA uncuttable by the restriction enzyme.
For each locus evaluated, T1 plants in which ZFNs were induced by estradiol were pooled together and genomic DNA was extracted. For the At1g53-ZFN, which targets the At1g53430-At1g53440 gene cluster, ZFN activity was detected at the At1g53430 target site (Figure S4A). Similarly, the At1g70-ZFN and At4g16-ZFN were found to be active at the At1g70450-At1g70460 gene cluster and the RPP4 gene cluster, respectively (Figure S4, B and C). We did not detect ZFN activity for the At3g21-ZFN and the At5g01-ZFN (Figure S3, data not shown). These data suggest that three of the five ZFN pairs are functional, and their activity can be temporally controlled by the estradiol-inducible promoter (Figure 1). We thus focused on the three active ZFNs for further study.
Quantification of ZFN activity at seven endogenous loci
We screened multiple T2 populations of Arabidopsis plants transformed with each ZFN pair and selected two independent lines showing high ZFN activity. These transgenic plants were used to quantify ZFN activity based on the level of NHEJ mutagenesis in whole seedlings. Note that the frequency of mutation observed by enrichment PCR is influenced by the position of the restriction enzyme site. The AflII and BfaI sites are very close to the middle of the spacer in the At1g70-ZFN and At4g16-ZFN recognition sites, respectively. In these cases, we reasoned the percentage of uncut DNA by these restriction enzymes should approximate the ZFN-mediated mutagenesis frequency. On the other hand, the DdeI site is farther away from the spacer in the At1g53-ZFN target site. Because only a fraction of mutagenesis events are likely assessed by measuring loss of the DdeI site, the T7 endonuclease assay was instead used to measure activity of the At1g53-ZFN. Activity of the three ZFNs at seven endogenous loci ranged from a few percent to 20% (Figure 2, A−C), with the At4g16-ZFN having the greatest activity (13.4–20.1%). Differences observed in ZFN activity among different transgenic plants is likely due to different expression levels of the ZFN transgenes, depending on the chromosomal site of T-DNA integration.
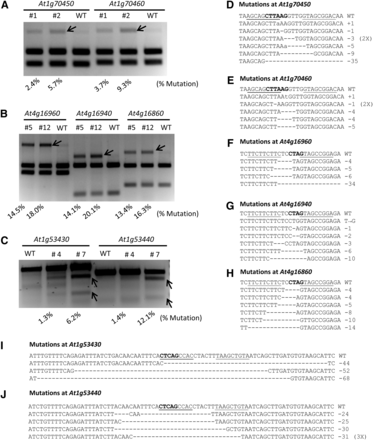
ZFN activity at seven endogenous loci. (A) At1g70-ZFN activity at target sites in At1g70450 and At1g70460. PCR products were digested with AflII. (B) At4g16-ZFN activity at target sites in At4g16960, At4g16940, and At4g16860. PCR products were digested with BfaI. (C) At1g53-ZFN activity at target sites in At1g53430 and At1g53440 as measured by the T7 endonuclease assay. For both restriction digestion assays (A and B) and the T7 endonuclease assay (C), mutagenesis frequencies (shown at the bottom of the figures) were determined by measuring the signal intensity of each band using the Labworks analysis software. (D-J) ZFN-induced mutations at seven endogenous target sites with different mutation types indicated. The restriction enzyme sites used for activity measurement are marked in bold letters. The uncut PCR products (from A and B, and Figure S2) were cloned and sequenced to reveal ZFN-induced mutations at each target site.
To further confirm activity of each ZFN, DNA fragments resistant to restriction enzyme digestion were cloned and sequenced (Figure 2, A and B). The sequencing results revealed ZFN-induced mutations at all seven target loci, with small deletions (1−10 bp) being the most prevalent (Figure 2, D−J). Because the DdeI restriction site is farther away from the spacer, deletions recovered at At1g53430 and At1g53440 were typically larger (24−68 bp; Figure 2, I and J and Figure S5).
Deletion of TAGs by ZFNs
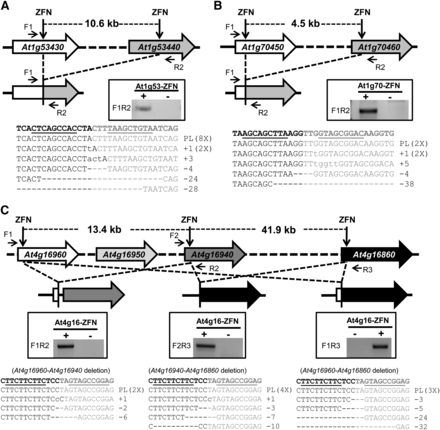
The observed mutations at all seven target sites indicate that ZFN-induced DSBs were generated. To examine deletion of TAGs as an outcome to ZFN activity, we conducted PCR using specific primers flanking all ZFN target sites, such that the production of PCR products would indicate the presence of deletions (Figure 3, A−C). With this strategy, we detected deletions of the At1g53430 gene cluster (Figure 3A) and the At1g70450 gene cluster (Figure 3B), as well as three different types of deletions at the RPP4 gene cluster (Figure 3C). We further confirmed these deletions by cloning the deletion-specific PCR products and sequencing randomly selected clones (Figure 3, A−C, lower panels). Not only did we observe indels at the site of deletions, we also observed perfect ligations of two sticky ends derived from ZFN-cleaved DNA (Figure 3, A−C). These events are most likely due to the presence of compatible overhangs. Taken together, these data suggest that deletions of up to 55 kb can be made by ZFNs on different Arabidopsis chromosomes.
Deletion of gene clusters by ZFNs. (A) Deletion of the At1g53430-At1g53440 cluster by the At1g53-ZFN. (B) Deletion of the At1g70450-At1g70460 cluster by the At1g70-ZFN. (C) Three types of deletions at the At4g16960-At4g16860 gene cluster generated by the At4g16-ZFN. The gene clusters and resulting deletions are depicted. Deletion events were confirmed by PCR, as shown in windows in the middle of each panel. The positions of PCR primers are indicated by arrows. PCR products were subsequently cloned and sequenced. The sequencing results shown in the lower panels confirmed perfect ligations after loss of the intervening DNA or ligations with mutations at the ZFN cleavage site.
Large chromosomal deletions by ZFNs
Because we achieved deletions of TAGs spanning up to 55 kb, we next tested whether much larger chromosomal deletions could be generated. To do so, we took advantage of an existing ZFN (ADH1-ZFN), which we previously engineered to target the ADH1 gene (At1g77120) at the end of chromosome 1 (Zhang et al. 2010) (Figure 4A and Figure S6A). Simultaneous expression of At1g53-ZFN and ADH1-ZFN could potentially result in chromosomal deletions as large as 9 Mb, almost one-third the length of Arabidopsis chromosome 1 (Figure 4A). We first screened independent estradiol-inducible ADH1-ZFN lines and identified the ADH1-ZFN #3 line as showing strong estradiol-inducible mutagenesis activity (Figure S6B). We also mapped the ADH-ZFN transgene insertion site in this line to chromosome 2 to aid in genotyping (Figure S6C). We then obtained a homozygous T3 ADH1-ZFN #3 line for use in our experiments.
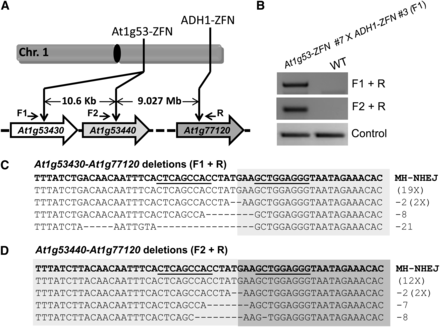
Large chromosomal deletions by ZFNs. (A) Schematic of ZFN targets on the right arm of Arabidopsis chromosome 1. The distance between the ZFN sites is shown and the positions of primers used to confirm large deletions are indicated. (B) PCR confirmation of large chromosomal deletions. The F1 and R primers amplify the junction fragment of the deletion of 9.037 Mb; primers F2 and R amplify the junction fragment of the deletion of 9.027 Mb (upper panels). PCR amplification of a part of the ADH1 gene was used as a genomic DNA control (lower panel). F1 seedlings generated from the cross between At1g53-ZFN #7 line and ADH1ZFN #3 line were treated with estradiol, and the wild-type plants served as a negative control. (C) Sequenced clones indicative of large chromosomal deletions between At1g53430 and At1g77120. (D) Sequenced clones indicative of large chromosomal deletions between At1g53440 and At1g77120. The DNA sequences resulting from perfect ligation of DNA ends are shown in the first line of the text boxes; deletions with indels are shown below. ZFN binding sequences are underlined. MH-NHEJ, end-joining that appears to have been facilitated by microhomology.
To test for chromosomal deletions, we crossed the homozygous At1g53-ZFN #7 T2 line and the ADH1-ZFN #3 T3 line. F1 plants were obtained, and both ZFNs were induced by growing them on estradiol-containing MS medium. As with the gene cluster deletions, PCR was used to detect the large chromosomal deletions, and deletions were detected for both At1g53430-At1g77120 and At1g53440-At1g77120 (Figure 4B). Resulting PCR products were cloned and sequenced (Figure 4, C and D). Interestingly, the most prevalent product was a ligation of a 7-bp spacer sequence. We predict this product resulted from microhomology-based NHEJ using only 1 bp of microhomology (Figure S7). The resulting 7-bp spacer would be expected to be cut inefficiently by the hybrid ZFN, which contains At1g53-ZFN-left and ADH1-ZFN-right monomers (Figure S7). This may explain the PCR product’s predominance.
Frequency of ZFN-induced chromosomal deletions
Having demonstrated ZFN-induced chromosomal deletions ranging from a few kilobases to 9 Mb, we next sought to estimate their frequency of occurrence. We adapted a digital PCR method used to measure deletion frequencies in human cells (Lee et al. 2010). In our case, deletion frequency was detected by PCR using a series of genomic DNA dilutions as templates. Amplification of ADH1 was used as an internal control for DNA copy number. As summarized in Table 1, the frequency of gene cluster deletions was approximately 1%—positively correlated with ZFN activity and negatively correlated with the length of the deletions. As the length of deletion increased to 9 Mb, the frequency decreased to <0.1%.
Frequency of ZFN-induced chromosomal deletions
| Transgenic Lines . | At1g53430 to At1g53440 (deletion of 10.6 kb) . | At1g70450 to At1g70460 (deletion of 4.5 kb) . | At4g16940 to At4g16860 (deletion of 41.9 kb) . | At4g16960 to At4g16860 (deletion of 55.3 kb) . | At1g53440 to At1g77120 (deletion of 9.027 Mb) . | At1g53430 to At1g77120 (deletion of 9.037 Mb) . |
|---|---|---|---|---|---|---|
| A1g53-ZFN #4 | 0.3% | |||||
| At1g53-ZFN #7 | 1% | |||||
| At1g70-ZFN #1 | 3% | |||||
| At1g70-ZFN #2 | 3% | |||||
| At4g16-ZFN #5 | 1% | 1% | ||||
| At4g16-ZFN #12 | 3% | 3% | ||||
| ADH1-ZFN #3 X | 0.046% | 0.137% | ||||
| At1g53-ZFN #7a |
| Transgenic Lines . | At1g53430 to At1g53440 (deletion of 10.6 kb) . | At1g70450 to At1g70460 (deletion of 4.5 kb) . | At4g16940 to At4g16860 (deletion of 41.9 kb) . | At4g16960 to At4g16860 (deletion of 55.3 kb) . | At1g53440 to At1g77120 (deletion of 9.027 Mb) . | At1g53430 to At1g77120 (deletion of 9.037 Mb) . |
|---|---|---|---|---|---|---|
| A1g53-ZFN #4 | 0.3% | |||||
| At1g53-ZFN #7 | 1% | |||||
| At1g70-ZFN #1 | 3% | |||||
| At1g70-ZFN #2 | 3% | |||||
| At4g16-ZFN #5 | 1% | 1% | ||||
| At4g16-ZFN #12 | 3% | 3% | ||||
| ADH1-ZFN #3 X | 0.046% | 0.137% | ||||
| At1g53-ZFN #7a |
ZFN, zinc finger nucleases.
F1 plants.
| Transgenic Lines . | At1g53430 to At1g53440 (deletion of 10.6 kb) . | At1g70450 to At1g70460 (deletion of 4.5 kb) . | At4g16940 to At4g16860 (deletion of 41.9 kb) . | At4g16960 to At4g16860 (deletion of 55.3 kb) . | At1g53440 to At1g77120 (deletion of 9.027 Mb) . | At1g53430 to At1g77120 (deletion of 9.037 Mb) . |
|---|---|---|---|---|---|---|
| A1g53-ZFN #4 | 0.3% | |||||
| At1g53-ZFN #7 | 1% | |||||
| At1g70-ZFN #1 | 3% | |||||
| At1g70-ZFN #2 | 3% | |||||
| At4g16-ZFN #5 | 1% | 1% | ||||
| At4g16-ZFN #12 | 3% | 3% | ||||
| ADH1-ZFN #3 X | 0.046% | 0.137% | ||||
| At1g53-ZFN #7a |
| Transgenic Lines . | At1g53430 to At1g53440 (deletion of 10.6 kb) . | At1g70450 to At1g70460 (deletion of 4.5 kb) . | At4g16940 to At4g16860 (deletion of 41.9 kb) . | At4g16960 to At4g16860 (deletion of 55.3 kb) . | At1g53440 to At1g77120 (deletion of 9.027 Mb) . | At1g53430 to At1g77120 (deletion of 9.037 Mb) . |
|---|---|---|---|---|---|---|
| A1g53-ZFN #4 | 0.3% | |||||
| At1g53-ZFN #7 | 1% | |||||
| At1g70-ZFN #1 | 3% | |||||
| At1g70-ZFN #2 | 3% | |||||
| At4g16-ZFN #5 | 1% | 1% | ||||
| At4g16-ZFN #12 | 3% | 3% | ||||
| ADH1-ZFN #3 X | 0.046% | 0.137% | ||||
| At1g53-ZFN #7a |
ZFN, zinc finger nucleases.
F1 plants.
Targeted inversions of gene clusters
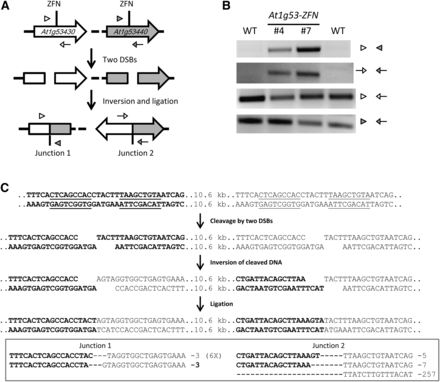
In addition to deletions, chromosomal DNA released by two DSBs could create inversions (illustrated in Figure 5A). Such inversions will have two novel junction sites, which can be detected with PCR using specific primer sets (Figure 5A). The predicted novel junctions were, in fact, detected at the At1g53430 gene cluster by PCR; DNA sequencing confirmed that the inversions occurred (Figure 5B). As anticipated, many inversion junctions had small deletions indicative of imprecise NHEJ (Figure 5C). We could also detect and confirm inversions at the At1g70450 gene cluster (Figure S8), and these occurred at a frequency of ~0.05% as measured by digital PCR. Gene cluster inversions, therefore, appear to occur at a lower frequency than deletions.
Inversion of the At1g53430 gene cluster. (A) Schematic of the At1g53430-At1g53440 gene cluster inversion. Positions of PCR primers for confirmation of inversions are indicated by empty or filled triangles and arrows. (B) PCR confirmation of gene cluster inversions. Two independent T2 lines were used to detect inversions; wild-type (WT) plants were used as the negative control. (C) Detailed depiction of the inversion event and DNA sequence confirmation of the inversions.
Possible targeted duplication of gene clusters
When two DSBs are induced in different TAGs on different chromosomes (either homologous chromosomes or sister chromatids), interchromosomal ligations of the broken DNA ends can result in deletions as well as gene cluster duplications (Figure S4A). For TAGs that only contain two genes, such as the At1g70450 gene cluster, the duplication will create a hybrid gene (Figure S9A). We indeed detected specific products indicative of ZFN-induced duplications of the At1g70450 gene cluster (Figure S9C), and these were also confirmed by DNA sequencing of the cloned PCR products (Figure S9D). However, if deleted DNA was circularized and retained in cells, it would give the same PCR products as depicted in Figure S9B. Because we cannot distinguish between these two outcomes, and because we do not know how long DNA circles persist in cells, it remains unclear how many of the events detected (Figure S9, C and D) truly reflect gene cluster duplications.
Discussion
We previously reported engineering active ZFNs for six endogenous loci in Arabidopsis using the CoDA method (Sander et al. 2011). In this study, we successfully targeted seven additional loci with three ZFNs, each of which showed NHEJ mutagenesis activity. Two other ZFNs targeting different endogenous loci failed to show detectable activity in Arabidopsis. For these ZFNs, it remains possible that chromatin structure or epigenetic modifications impeded ZFN access to endogenous DNA targets. Our overall 60% success rate is in line with the success rate previously reported for CoDA, and our work further demonstrates the usefulness of the CoDA method for engineering active ZFNs.
We aimed to create deletions of TAGs in the Arabidopsis genome by using a single ZFN pair to target multiple genes within the cluster. However, not every gene cluster can be deleted with such a strategy. In cases in which the TAGs differ significantly in DNA sequence similarity, two different pairs of ZFNs might be required to create the desired deletion (Sollu et al. 2010). In addition, TALENs are good alternatives to ZFNs, because there seems to be less restriction in designing TALENs to target diverse DNA sequences (Bogdanove and Voytas 2011; Doyle et al. 2012; Reyon et al. 2012). The scale of deletions that we obtained (from a few kilobases to 9 Mb) suggests ZFNs are capable of creating very large deletions in plants. We recognize, however, that Arabidopsis plants are not likely to survive the loss of megabase pairs of chromosomal DNA. Targeted insertions created by HR were previously demonstrated by the use of ZFNs in plants (Cai et al. 2009). Generating deletions (such as in this study) and concomitantly creating insertions by HR may be another genome engineering approach of value for basic research and crop improvement.
Being highly homologous and repetitive, TAGs are particularly prone to change either through unequal crossover or gene conversion. This provides the opportunity to evolve new gene functions, some of which may be adaptive (Hanada et al. 2008; Lehti-Shiu et al. 2009). Our approach for making deletions and duplications in TAGs has demonstrated that we can now create novel chimeric genes which may otherwise not occur naturally. For example, we frequently detected hybrid genes due to perfect ligation of broken chromosomes after loss of the intervening DNA (Figure 3, A−C). In addition, duplication of gene clusters increases genetic redundancy and frees some gene members to evolve new functions. Tandem duplication in Arabidopsis has provided a means for adaptive evolution of R genes (Meyers et al. 2005). Our approach in creating rearrangements in the complex RPP4 gene cluster and the two RLK gene clusters is thus a promising first step toward generating valuable genetic material for both molecular and evolutionary studies.
Gene cluster inversion is another consequence of our TAG-targeting approach. For TAGs encoding two genes, DNA inversion is likely to destroy the function of both gene targets. However, because inversions preserve DNA sequences (which would otherwise be lost in deletions), they may have unique applications, such as serving as templates for future genome evolution. Another application of inversions is that they may only knock out two target genes at both ends of the TAG while retaining the function of the genes in between. This is only true if there are more than two members in the TAG, such as the RPP4 gene cluster evaluated in this study.
To recover plants with germline-transmitted deletions, we screened large T3 populations of At1g70-ZFN #2 (a total of 2539 plants) and At4g16-ZFN #12 (a total of 2322 plants) with no success (data not shown). Clearly, the frequencies of germline-transmitted deletions are much lower than the somatic frequencies. This observation is consistent with other ZFN-mediated mutagenesis studies we have conducted (unpublished data), where the frequency of somatic mutation did not directly reflect the frequency of germline mutation. Rather, we believe there is a threshold somatic mutation frequency that must be surpassed to ensure successful germline transmission. In previous work, we found that somatic mutagenesis frequencies in excess of 7% were sufficient to recover germinal mutations at high frequency (Zhang et al. 2010). The observed ~1% somatic deletion frequency observed here appears to be under this threshold.
It has been shown that stem cell niches in Arabidopsis are hypersensitive to DNA damage, and an even a low dose of DNA damage can trigger programmed cell death selectively in these stem cells (Fulcher and Sablowski 2009). Interestingly, programmed cell death in the shoot meristem was greatly suppressed when the DNA damage early response gene, ATM, was knocked out. Thus, it might be useful to use an atm mutant background to screen for germline deletions in plants. In addition, we recently reported that smc6b mutations promote NHEJ in Arabidopsis (Qi et al. 2013). As chromosomal breaks that lead to deletions are joined through NHEJ, it is likely that smc6b mutations will enhance deletion frequencies. It is also possible some deletions are deleterious to pollen and egg cells, and this impedes their transmission. Because whole plant regeneration routinely is performed from somatic cells for many species (e.g., rice, maize, and tobacco), plants with deletions may be easier to achieve by regenerating somatic tissues that have been treated with sequence specific nucleases.
Note Added in Proof: See also Michelle Christian, Yiping Qi, Yong Zhang, and Daniel F. Voytas, 2013 Targeted Mutagenesis of Arabidopsis thaliana using Engineered TAL Effector Nucleases (TALENs) G3: Genes, Genomes, Genetics 3: 1697–1705.
Acknowledgments
We thank all members of the Voytas lab for helpful discussions and suggestions. This work is supported by grants from the National Science Foundation (MCB 0209818 and DBI 0923827) to D.F.V. and a National Institutes of Health (NIH) Director’s Pioneer Award DP1 GM105378 (J.K.J.) and NIH R01 GM088040 to J.K.J. D.F.V. has a financial interest in and serves as Chief Science Officer of Cellectis Plant Sciences. These interests have been reviewed and managed by the University of Minnesota in accordance with its Conflict of Interest policies. J.K.J. has a financial interest in Transposagen Biopharmaceuticals. J.K.J.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Footnotes
Communicating editor: K. M. Devos
Literature Cited
Author notes
Supporting information is available online at http://www.g3journal.org/lookup/suppl/doi:10.1534/g3.113.006270/-/DC1
Present address: Cellectis Plant Sciences, 600 County Road D, West Suite 8, New Brighton, MN 55112.